Pharmaceutical market research companies for compliance
-
Bella Williams
- 10 min read
Compliance Pharma Insights serve as a crucial element for understanding the evolving needs within the pharmaceutical industry. Conducting thorough market research helps companies identify areas of risk, regulatory challenges, and customer pain points. Engaging with these insights allows stakeholders to navigate complexities while ensuring adherence to compliance standards.
The importance of data accuracy cannot be overstated. Reliable insights derived from targeted research can significantly influence decision-making processes. As companies strive to remain compliant, they must prioritize gathering actionable insights that inform strategies and enhance their market positioning. Understanding Compliance Pharma Insights ultimately fosters a more informed and resilient pharmaceutical environment.
Importance of Compliance in Pharmaceutical Market Research
Compliance is a critical element in pharmaceutical market research, ensuring that data collection, analysis, and reporting adhere to legal and ethical standards. Organizations that prioritize compliance foster trust among stakeholders, including patients, healthcare providers, and regulatory bodies. This trust is essential for obtaining accurate data and conducting effective research while avoiding potential legal repercussions that can arise from non-compliance.
Adhering to compliance regulations also enhances the credibility of research findings. By maintaining rigorous standards, companies secure valuable Compliance Pharma Insights that guide strategic decision-making and improve overall product offerings. Ignoring compliance can jeopardize not only a study’s integrity but also a company's reputation. Thus, embracing compliance is not just a legal obligation; it is a smart business practice that drives sustainable growth and fosters a culture of accountability within the organization.
Regulatory Landscape and Compliance Requirements
Navigating the regulatory environment for pharmaceutical market research requires an understanding of compliance requirements. Compliance Pharma Insights reveal the importance of adhering to strict data privacy regulations, such as GDPR and HIPAA. Research firms must ensure that sensitive data remains secure and is processed in line with these regulations.
There are several key elements to consider when evaluating compliance in pharmaceutical market research. First, firms must maintain data residency, ensuring that data collected in Europe remains within its borders. Second, transparent communication about data usage enhances trust with clients and stakeholders. Finally, continuous monitoring of evolving regulations allows companies to adapt their practices and remain compliant. Familiarity with these compliance benchmarks is crucial for successfully operating within the pharmaceutical sector, ultimately fostering confidence and accountability in market research activities.
Role of Market Research in Ensuring Compliance
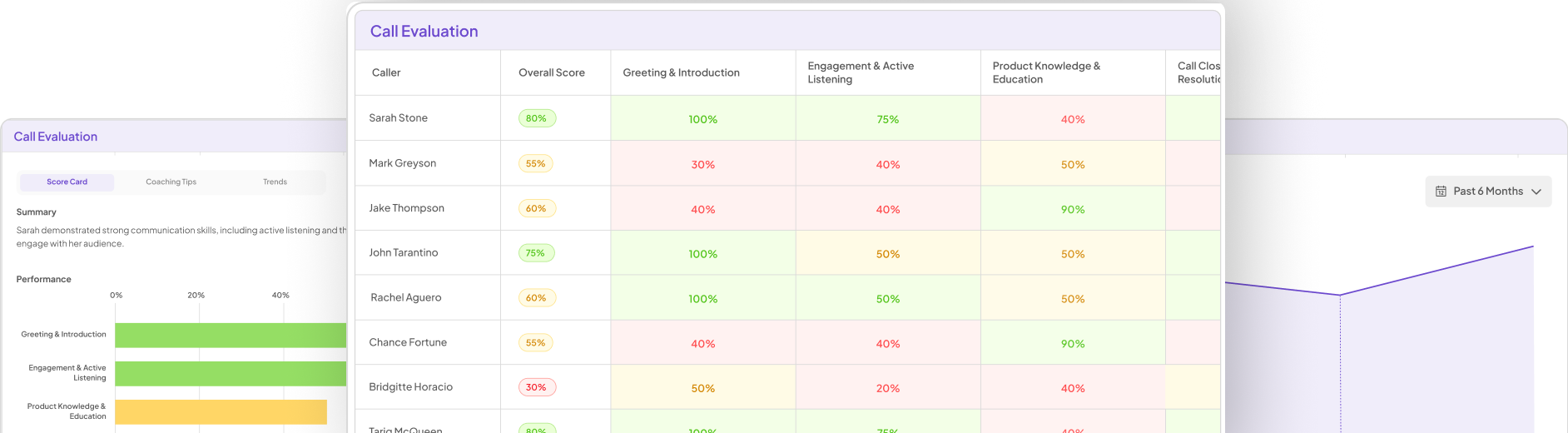
Market research plays a critical role in ensuring compliance within the pharmaceutical sector. It enables companies to collect valuable Compliance Pharma Insights that guide their decision-making processes. Understanding regulations and market dynamics is vital for pharmaceutical firms to align with compliance requirements. This proactive approach helps to mitigate risks associated with regulatory changes and improves overall operational efficiency.
Through comprehensive research, organizations can identify area-specific compliance challenges and customer needs. It aids in monitoring competitor activities, regulatory shifts, and emerging trends that may impact compliance standards. Regular analysis of market data equips companies with the insights necessary to adapt their strategies effectively. By prioritizing research-driven compliance efforts, pharmaceutical companies can foster trust and maintain their reputation in a highly regulated environment.
Leading Pharmaceutical Market Research Companies for Compliance Pharma Insights
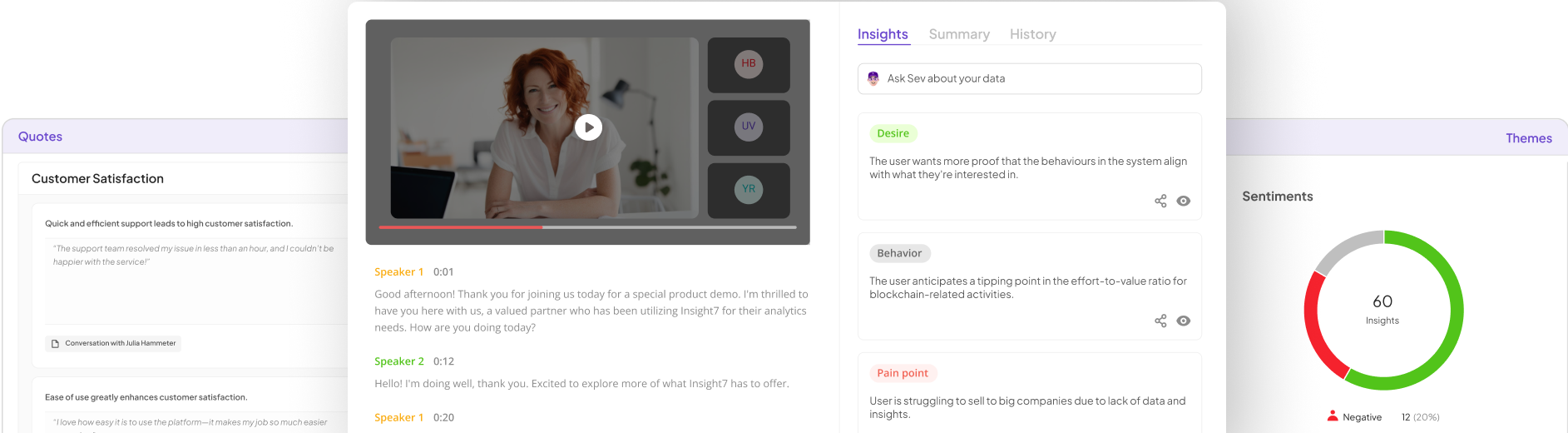
Leading pharmaceutical market research companies play a crucial role in providing Compliance Pharma Insights, which are essential for navigating the complex regulatory landscape of the industry. These companies utilize advanced methodologies to ensure accurate data collection, analysis, and reporting. They specialize in qualitative research, allowing teams to understand customer behavior deeply and accurately.
Furthermore, the use of AI-driven tools enhances the quality of insights, making it easier for organizations to identify compliance issues and mitigate risks. By focusing on credible data, these firms help pharmaceutical companies develop strategies that adhere to regulatory standards while fostering innovation. Ultimately, their contributions are invaluable in maintaining compliance and ensuring the effectiveness of pharmaceutical solutions in the market.
Top Companies Specialized in Compliance Research
Compliance research in the pharmaceutical industry is crucial for ensuring that companies adhere to regulatory standards and maintain product integrity. These specialized firms excel in delivering Compliance Pharma Insights that guide businesses through complex regulatory frameworks. They focus on collecting and analyzing data to identify compliance gaps, helping companies avoid costly fines and reputation damage.
Several key characteristics define these top compliance research firms. First, they possess deep industry expertise, enabling them to interpret regulations effectively. Next, they utilize advanced analytics tools to extract actionable insights from vast data sets. Additionally, these companies emphasize a collaborative approach, working closely with clients to tailor solutions. This partnership fosters a proactive compliance culture. Overall, selecting a reliable compliance research firm is essential for pharmaceutical companies aiming to stay ahead in a highly regulated environment.
Criteria for Selecting the Best Compliance Pharma Insights Partner
Selecting the best compliance pharma insights partner involves several crucial criteria. First, experience in the pharmaceutical sector and a robust understanding of regulatory compliance are essential. A partner must demonstrate expertise in delivering actionable insights tailored to compliance needs. This ensures that any market research conducted aligns with industry standards and regulations, fostering trust and reliability.
Next, evaluate the methodologies employed by potential partners. Strong analytical tools and techniques should be at their disposal to process data effectively. Their approach should include an emphasis on minimizing bias in user research, resulting in credible insights. Assess their track record in client satisfaction and their ability to provide timely results. By focusing on these aspects, organizations can secure a compliance pharma insights partner that not only meets regulatory demands but also drives informed decision-making.
Conclusion: The Future of Compliance Pharma Insights in Market Research
The future of compliance pharma insights in market research is poised for transformative change. As regulatory standards evolve, the integration of advanced technologies will streamline processes and enhance the accuracy of data analysis. Pharmaceutical market research companies will be tasked with navigating these shifts by ensuring diligent compliance while deriving actionable insights from qualitative research.
Moreover, the emphasis on secure data handling will strengthen trust between stakeholders in the industry. By embracing robust methodologies and innovative tools, organizations can unlock the potential of compliance pharma insights, fostering improved decision-making and strategic growth. Ultimately, the commitment to maintaining high standards will define the success of market research initiatives in this crucial sector.