Using AI in Clinical Trials: Best Practices
-
Bella Williams
- 10 min read
AI-Enhanced Trials are revolutionizing the approach to clinical research by streamlining processes and improving outcomes. Innovations in artificial intelligence are paving the way for more efficient trial designs, data management, and participant recruitment. As researchers seek to address the unique challenges of modern clinical trials, AI serves as a crucial tool for enhancing data analysis and decision-making.
Through AI-Enhanced Trials, the focus shifts toward improving patient experiences and outcomes. By utilizing advanced algorithms and machine learning, researchers can gain deeper insights from vast datasets, leading to more informed choices regarding trial methodologies. This transformative process not only boosts operational efficiency but also accelerates the path to regulatory approval and market readiness.
Best Practices for AI-Enhanced Trials
To implement AI-Enhanced Trials effectively, it is crucial to adopt certain best practices that streamline processes and improve accuracy. One important aspect is to ensure data quality from the outset, as the insights gained depend largely on the integrity of the input data. Engage with the stakeholders early on to align AI objectives with trial requirements. This proactive collaboration can lead to more tailored solutions that meet specific clinical needs.
Another best practice involves continuous training and adaptation of AI tools. As clinical trials evolve, so should the algorithms that power them. Regularly updating and retraining AI systems improves their performance and relevance in dynamic environments. Moreover, fostering a culture of transparency regarding AI outputs ensures that teams can trust the insights generated, leading to better decision-making. By following these best practices, organizations can harness the full potential of AI-Enhanced Trials, ultimately improving patient outcomes and efficiencies in the research process.
Data Collection and Management in AI-Enhanced Trials
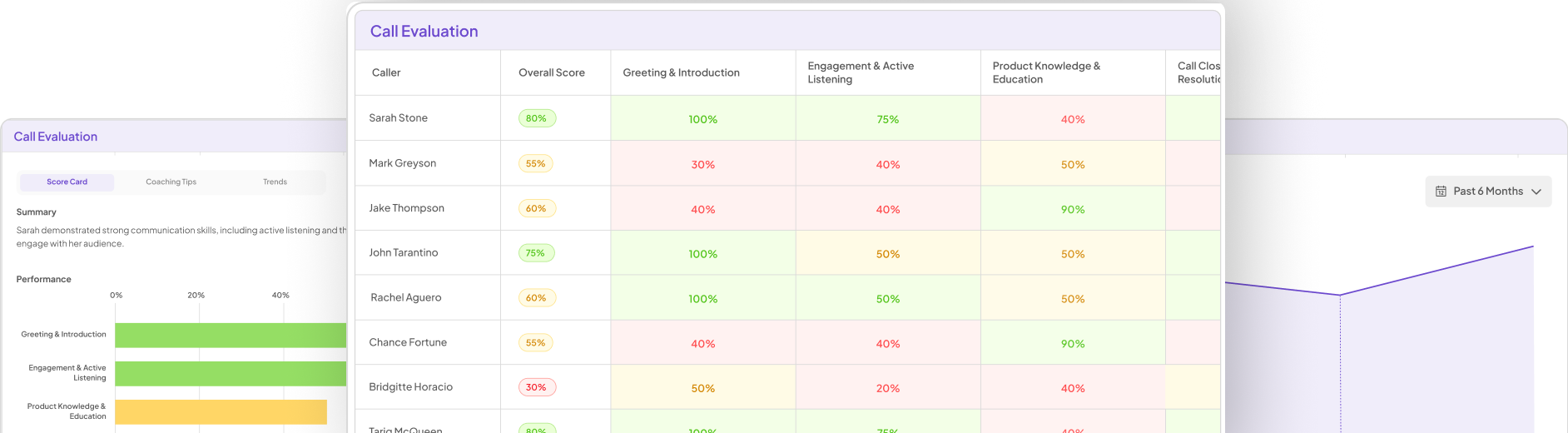
In AI-Enhanced Trials, effective data collection and management are pivotal for achieving reliable outcomes. Ensuring accurate data input is the first step, as it forms the foundation of all subsequent analyses. Participants’ information needs to be gathered in a structured manner, minimizing errors and inconsistencies. Automated tools can significantly aid this process, enhancing precision and efficiency.
Once collected, data management becomes essential. Proper organization of information allows researchers to easily access and analyze data, while maintaining compliance with ethical standards. Implementing robust security measures guarantees participant confidentiality, fostering trust throughout the trial process. Regular audits of data practices can further enhance reliability, allowing quick identification and rectification of any inconsistencies. Through these practices, AI-Enhanced Trials can lead to more robust findings and improved patient outcomes.
Leveraging Machine Learning Algorithms for AI-Enhanced Trials
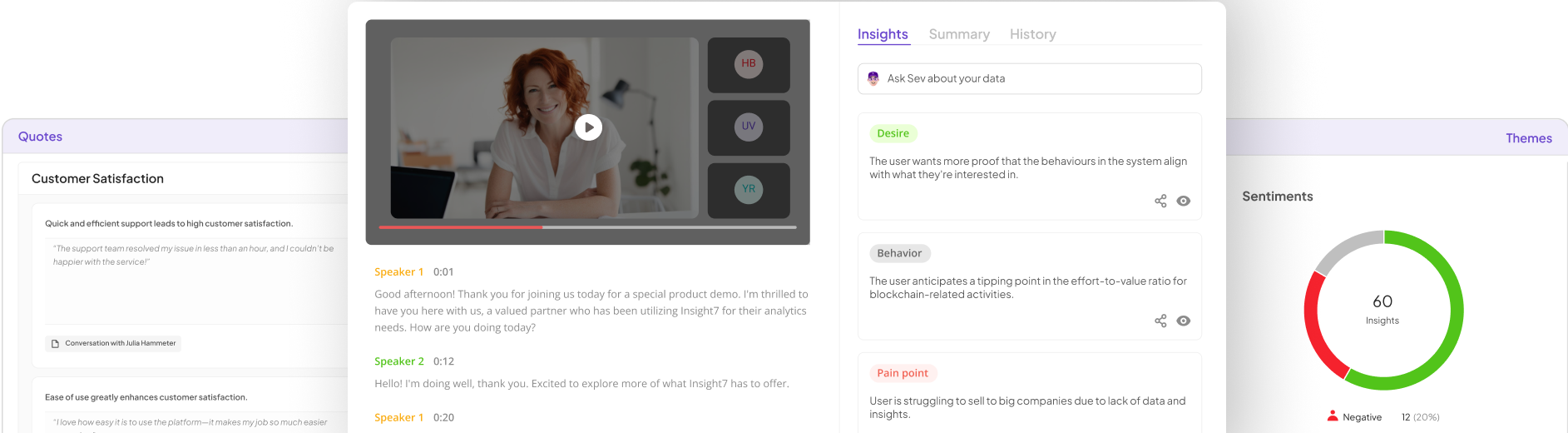
Machine learning algorithms play a critical role in enhancing clinical trials through data analysis and predictive modeling. These algorithms can process vast amounts of real-time data, identifying patterns that human reviewers might overlook. Consequently, leveraging machine learning helps streamline the trial process, improving efficiency and accuracy in reaching insights. By automating data analysis, researchers can focus on interpreting results and making informed decisions rather than getting bogged down in overwhelming data.
Moreover, machine learning can significantly reduce patient selection bias by analyzing demographic and historical data to identify suitable participants. This enhances patient recruitment, ensuring a representative sample that reflects real-world conditions. Ultimately, embracing these strategies leads to AI-enhanced trials that are faster, more precise, and aligned with the goals of advancing medical research. As the role of AI in clinical trials evolves, it offers tremendous potential for improved patient outcomes and data integrity.
Ensuring Ethical Standards in AI-Enhanced Trials
In AI-Enhanced Trials, maintaining ethical standards is crucial for the integrity of research outcomes. Transparency in data collection and algorithm application is vital to avoid bias. Stakeholders must prioritize informed consent, ensuring participants are fully aware of how AI influences their involvement and outcomes. This means clear communication about the role of AI tools in the trials and potential risks.
Additionally, continuous monitoring of AI systems helps detect and rectify any emerging ethical issues. Regular audits of data usage and decision-making processes will ensure compliance with ethical guidelines. Engaging diverse perspectives during the design and implementation phases also contributes to more equitable AI applications. Ultimately, fostering trust through ethical practices will enhance the credibility of AI-Enhanced Trials and lead to better healthcare solutions.
Data Privacy Concerns in AI-Enhanced Trials
Data privacy is increasingly crucial in AI-enhanced trials. As these trials utilize vast amounts of sensitive patient data, concerns regarding unauthorized access and data breaches arise. Protecting the confidentiality and integrity of patient information must be a top priority for all stakeholders involved.
To address these concerns, several key strategies can be adopted. First, adherence to regulatory frameworks, such as GDPR, helps ensure compliance and promotes trust among participants. Second, implementing robust encryption methods safeguards data during transmission and storage. Third, continuous risk assessments identify potential vulnerabilities, allowing teams to adapt and improve their security measures. Lastly, involving patients in the conversation fosters transparency, as sharing how data is used can enhance trust and participation in AI-enhanced trials. By prioritizing these practices, stakeholders can navigate the complexities of data privacy while reaping the benefits of innovative research methods.
Addressing Bias and Fairness in AI-Enhanced Trials
AI-Enhanced Trials present unique challenges in addressing bias and ensuring fairness. Bias can infiltrate various stages of a clinical trial, from participant selection to data interpretation. Acknowledging potential biases is crucial for trial integrity and outcomes. Relying solely on historical data can perpetuate these biases, as the results may not reflect the broader demographic. Applying rigorous methodologies to recognize and mitigate bias is essential in creating a fair testing environment.
To effectively address bias in AI-Enhanced Trials, several key strategies should be considered. First, implementing diverse data sets can help ensure that various population segments are accurately represented. Second, algorithms must be regularly audited to identify any patterns of discrimination or inaccuracies in predictions. Third, stakeholder engagement, particularly from underrepresented communities, can provide insights that help refine trial designs. Lastly, continuous education on bias and its implications for AI can empower teams to make informed decisions throughout the trial process. By actively working towards fairness, we can enhance trust in AI-enhanced clinical trials and their results.
Conclusion: The Future of AI-Enhanced Trials in Clinical Research
The future of AI-Enhanced Trials in clinical research holds significant promise. As technology continues to evolve, clinical trials will become increasingly streamlined and efficient, allowing for faster drug development. AI can play a vital role in improving data collection and analysis, potentially decreasing the time it takes to gather vital research insights.
Collaboration between AI tools and researchers will drive innovative methodologies in trial design. Such advancements not only enhance participant engagement but also ensure more robust results. By embracing AI-Enhanced Trials, the clinical research community can pave the way for a new era of evidence-based medicine that is both ethical and effective.