How to Conduct QA in Highly Regulated Environments
-
Bella Williams
- 10 min read
Regulatory QA Practices form the backbone of quality assurance in highly regulated environments, where compliance is non-negotiable. Such settings, including healthcare and finance, require meticulous attention to established standards. Understanding these specialized practices is essential for professionals involved in quality assurance roles. These practices not only safeguard regulatory adherence but also enhance overall product safety and effectiveness.
Incorporating regulatory QA practices helps organizations navigate complex compliance landscapes while promoting a culture of quality. Effective strategies involve understanding relevant regulations, implementing structured processes, and utilizing the right tools for assessment. By prioritizing these elements, businesses can maintain rigorous quality standards and meet the demands of their respective industries successfully.
In the world of quality assurance, operating within highly regulated environments presents unique challenges and opportunities. This blog post will guide you through the best practices and strategies for conducting QA in these settings, ensuring compliance and maintaining high standards.
Operating within highly regulated environments presents both challenges and opportunities for quality assurance professionals. Regulatory QA practices require a keen understanding of the stringent guidelines and protocols that govern industries such as pharmaceuticals, finance, and healthcare. These environments demand meticulous attention to detail, as non-compliance can lead to severe consequences, including hefty fines and reputational damage. Thus, professionals must navigate these complexities with a strategic mindset while ensuring that quality standards remain high.
To effectively conduct QA in such settings, it is crucial to implement best practices that prioritize compliance and operational efficiency. These may include regularly updating training protocols, employing robust documentation practices, and conducting frequent audits. Establishing a culture of continuous improvement can also transform potential hurdles into opportunities for growth and innovation. This blog post will outline essential strategies for Regulatory QA Practices that not only guarantee compliance but also elevate your organization’s standards of quality.
In highly regulated environments, understanding regulatory QA practices is crucial for ensuring compliance and quality assurance. Navigating the intricate landscape of regulations requires a detailed examination of industry-specific requirements. Professionals should constantly assess the nuances of these rules, prioritizing clarity and accuracy in their reporting. By fostering open communication around regulatory expectations, organizations can better meet compliance criteria and avert costly penalties.
Equally important is the integration of risk management strategies within QA processes. Identifying potential risks early on facilitates proactive mitigation, minimizing the chances of compliance breaches. Emphasizing training and education ensures that team members are fully equipped to interpret regulatory mandates accurately. By intertwining regulatory QA practices with ongoing team development, organizations can secure a strong foundation for successfully navigating the complexities of compliance in demanding environments.
Analyze & Evaluate Calls. At Scale.
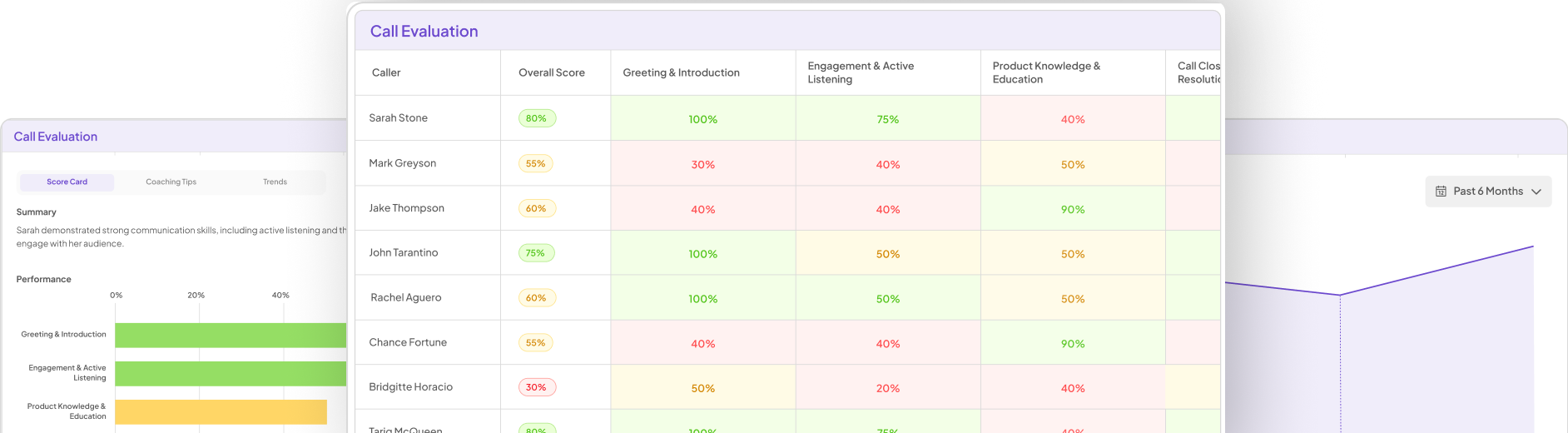
Key Considerations for Regulatory QA Practices
To ensure effective Regulatory QA Practices in highly regulated environments, several key considerations must be addressed. Firstly, professionals should familiarize themselves with the specific regulations that impact their industry. This understanding leads to better compliance and enhances quality assurance processes. Regular training and updates on these regulations are essential for staying informed and competent.
Secondly, implementing a robust risk management framework is vital. This involves identifying potential risks associated with compliance and developing strategies to mitigate them. Effective risk management not only protects organizational interests but also fosters a culture of quality and accountability. Lastly, consider the tools available to support QA efforts. Utilizing technology can streamline processes and aid teams in adhering to regulatory standards, thereby reinforcing a commitment to quality assurance. With these considerations in mind, effective Regulatory QA Practices can be established and maintained.
When dealing with regulatory QA, its essential to understand the crucial factors that can impact the process. This section will cover the main considerations for ensuring effective quality assurance.
In the realm of regulatory QA, it is imperative to grasp the crucial factors influencing the quality assurance process. Understanding regulatory requirements is vital, as each industry is governed by specific compliance regulations. Professionals must not only identify these requirements but also continuously monitor updates, as regulations often evolve. Failing to comply can lead to significant repercussions, including fines and damage to reputation.
Moreover, effective risk management is a cornerstone of successful Regulatory QA Practices. Organizations need to identify and assess potential risks associated with compliance and quality issues. By implementing proactive strategies to mitigate these risks, quality assurance teams can preserve the integrity of their processes and products. In summary, a thorough understanding of regulatory requirements, coupled with robust risk management strategies, ensures effective quality assurance in highly regulated environments. Engaging in these practices fosters both compliance and excellence in quality outcomes.
Understanding Regulatory Requirements
To succeed in Regulatory QA Practices, professionals must grasp the specific regulations that govern their industry or product. Understanding these regulatory requirements involves not just reading through compliance texts but interpreting them in the context of your operations. Gathering relevant statutes, guidelines, and standards is the first step towards ensuring that your QA processes align with the law.
Once the regulations are identified, ongoing communication with regulatory bodies can clarify expectations. Regularly updating your knowledge base is essential as regulations often evolve. Furthermore, engaging with compliance experts can bring valuable insights into applying these regulations effectively within your QA frameworks. By comprehensively understanding these requirements, organizations can mitigate risks and enhance their quality assurance processes, ultimately supporting a culture of compliance and quality.
To succeed in QA, professionals must thoroughly comprehend the specific regulatory requirements applicable to their industry or product. We will explore how to gather and interpret these regulations effectively.
Quality assurance in highly regulated environments demands a comprehensive understanding of regulatory requirements specific to each industry. Professionals must familiarize themselves with applicable laws, guidelines, and standards, as these regulations guide the QA process and ensure compliance. Effective interpretation of these regulatory mandates not only mitigates risks but also enhances operational efficiency by integrating compliance measures seamlessly into the QA workflow.
To gather and interpret these regulations effectively, professionals can utilize several strategies. First, they should engage with relevant regulatory bodies and follow updates on regulations affecting their products or services. Second, industry-related organizations often publish insights that clarify obligations and best practices for maintaining compliance. Third, participating in training and workshops can also equip QA professionals with the tools needed to navigate complex regulations. By embracing these approaches, organizations position themselves to maintain the integrity of Regulatory QA Practices while fostering a culture of continuous improvement.
Risk Management in QA
In highly regulated environments, effective risk management in QA is indispensable for maintaining compliance and achieving quality standards. A structured approach allows organizations to identify potential risks early, enabling proactive measures to mitigate them. The first step in this process involves identifying risks associated with compliance and quality issues. This could include factors such as equipment failure, human error, or regulatory non-compliance that might impact overall quality assurance.
Next, risk analysis becomes crucial. This process helps to assess the likelihood and potential impact of each identified risk. By prioritizing these risks, organizations can focus on strategies that are most needed to mitigate them effectively. Finally, ongoing risk management is vital, as it promotes continuous monitoring and adjustment to ever-changing regulations and operational challenges. Achieving mastery in these areas not only ensures regulatory QA practices are adhered to but also enhances organizational resilience and product reliability in the long run.
Implementing a robust risk management strategy is vital in highly regulated environments. This sub-section will elaborate on identifying, analyzing, and mitigating risks associated with compliance and quality issues.
In highly regulated environments, a robust risk management strategy is indispensable. The first step involves identifying potential risks associated with compliance and quality issues. This requires a comprehensive understanding of regulatory standards and the specific requirements they impose. Regular assessments and audits can help pinpoint vulnerabilities and ensure that the organization remains aligned with legal obligations.
Once risks are identified, analyzing their potential impact is critical. A thorough risk analysis not only encompasses the likelihood of occurrence but also the severity of consequences if they materialize. This analysis can provide valuable insights that inform mitigation strategies. Mitigation efforts may involve implementing rigorous training programs, enhancing communication, or adopting advanced technology for monitoring and compliance.
By effectively identifying, analyzing, and addressing risks, organizations can ensure compliance with regulations while maintaining high-quality standards in their operations. This proactive approach is paramount to sustaining trust and credibility in highly scrutinized industries.
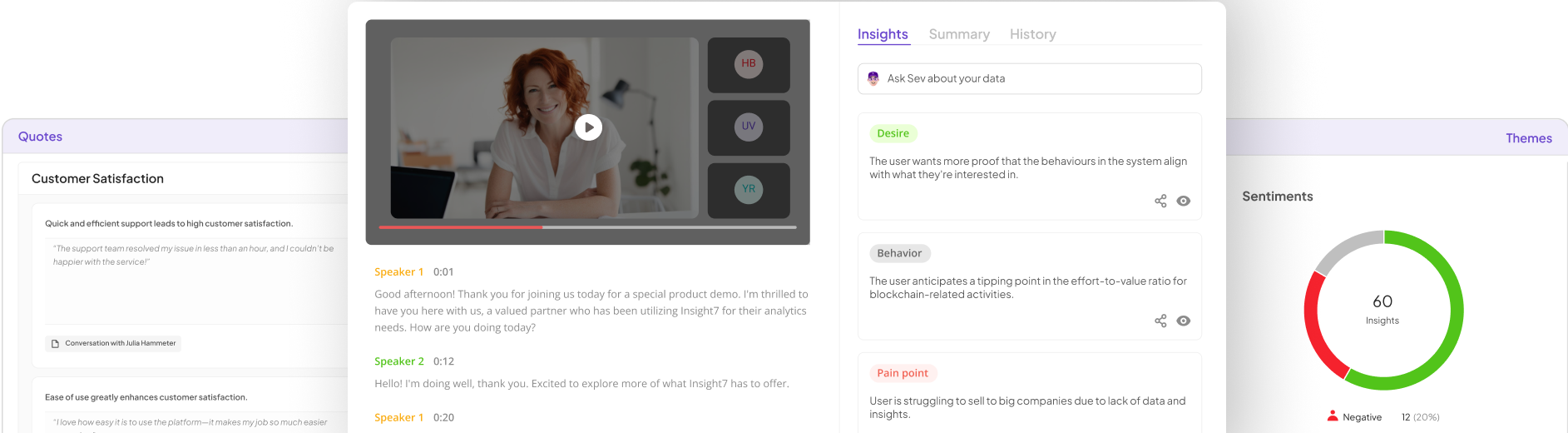
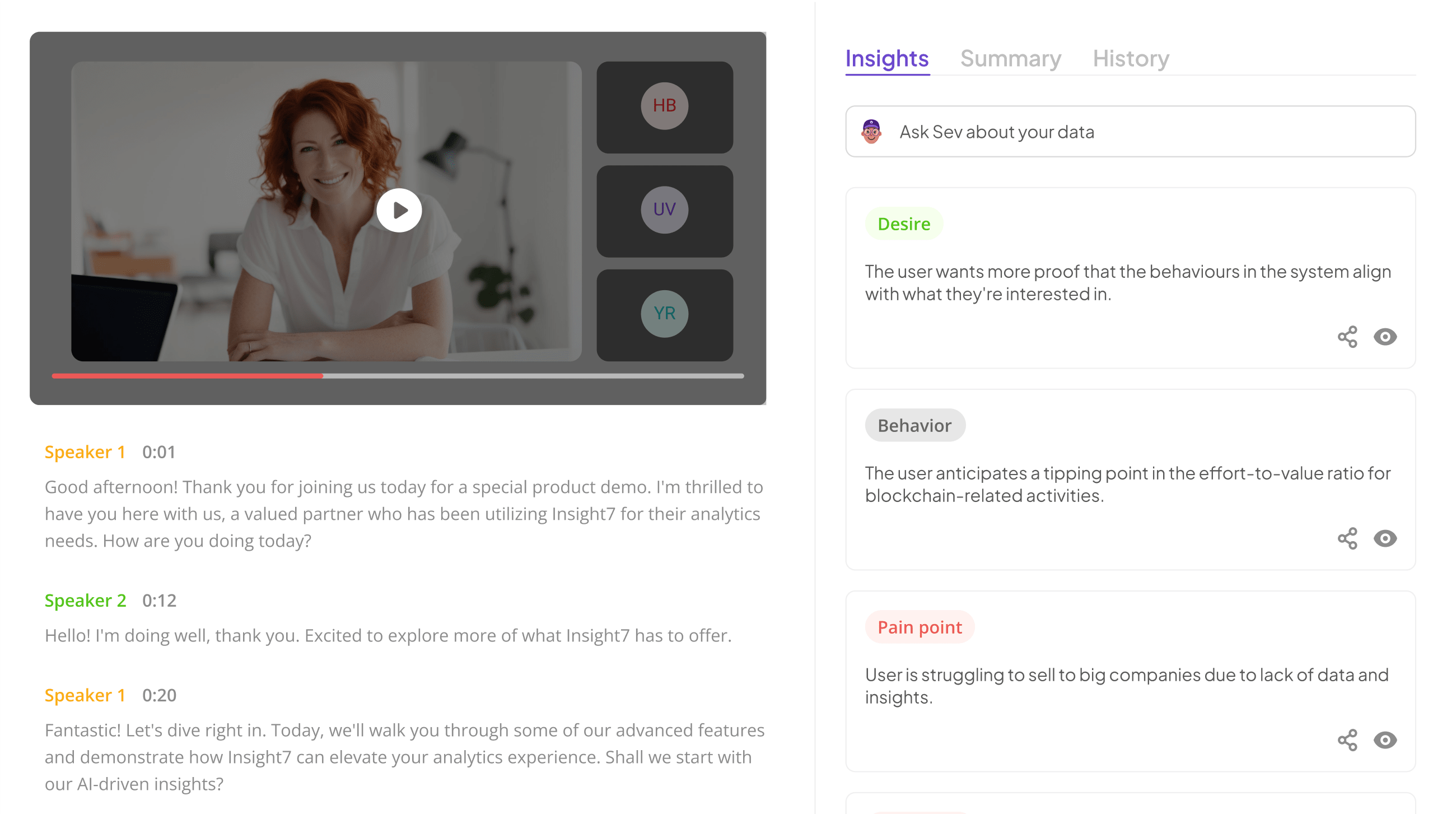
Extract insights from interviews, calls, surveys and reviews for insights in minutes
Essential Tools for Regulatory QA Practices
In regulatory QA practices, having the right tools is essential for ensuring compliance and maintaining quality. Tools specifically designed for regulated environments can significantly enhance the QA process by streamlining workflows, facilitating documentation, and improving communication among teams. By incorporating these essential tools, organizations can effectively manage their quality assurance efforts while adhering to the strict regulatory standards that govern their operations.
Among the top tools available, insight7 stands out as a comprehensive solution that simplifies the management of QA tasks. Its features enable users to easily record, transcribe, and analyze data, ensuring accurate evaluations against compliance criteria. Similarly, Jira excels in tracking workflows, making it easier to manage projects while keeping regulatory standards in focus. Other noteworthy tools include Zephyr, which enhances testing through effective management, HP ALM, which integrates development and QA processes, and QTest, which focuses on collaboration and test case management. Together, these tools form a robust toolkit for effective regulatory QA practices in highly controlled environments.
The right tools can significantly streamline the QA process in regulated environments. This section provides an overview of top tools that can aid in regulatory compliance and quality assurance.
In regulated environments, selecting the right tools is crucial for enhancing efficiency and compliance in the QA process. Regulatory QA practices benefit significantly from software solutions that provide streamlined workflows, improve communication, and ensure adherence to standards. A robust toolset facilitates accurate data management, reduces errors, and supports thorough documentation, all of which are necessary for maintaining compliance.
Several standout tools can assist in these endeavors. Insight7 stands out for its comprehensive features tailored to manage quality assurance effectively. It allows users to record, transcribe, and analyze calls against compliance templates, ensuring adherence to regulatory requirements. Jira enhances project tracking, allowing teams to monitor QA processes meticulously. Zephyr and HP ALM provide testing and application lifecycle management solutions that align with high-stakes regulations. Lastly, QTest fosters collaboration and manages test cases efficiently, supporting teams in maintaining adherence to essential regulatory frameworks. Together, these tools create a solid foundation for effective quality assurance in regulated environments.
Top QA Tools for Regulated Environments
Quality assurance in regulated environments demands specialized tools to ensure compliance and maintain the highest standards. The right software can streamline workflows, manage documentation, and improve testing processes crucial for adhering to regulatory QA practices. Selecting tools designed specifically for these settings enhances efficiency and reduces the risk of non-compliance.
One standout tool is insight7, which offers an all-in-one solution for recording, transcribing, and analyzing data efficiently. Its user-friendly interface allows teams to conduct thorough evaluations against pre-defined compliance templates. Another key player is Jira, renowned for its tracking capabilities that keep QA efforts aligned with regulatory standards throughout project lifecycles. Additionally, Zephyr provides a streamlined testing framework, while HP ALM combines application lifecycle management with quality assurance to meet stringent regulations. Lastly, QTest enables effective test case management, promoting collaboration and adherence to compliance frameworks. Each of these tools plays a vital role in supporting effective regulatory QA practices.
In this part, we discuss some of the leading tools designed to enhance QA efforts:
Implementing effective quality assurance (QA) in highly regulated environments demands the right tools tailored to meet stringent compliance standards. In this part, we will discuss some of the leading tools designed to enhance QA efforts, providing insights into their unique capabilities and applications.
First, insight7 stands out as a comprehensive solution that simplifies QA management while ensuring adherence to regulatory requirements. Its features allow users to efficiently analyze large datasets, making it an ideal choice for professionals navigating complex compliance landscapes. Second, Jira is widely respected for its robust tracking capabilities, helping teams monitor project progress and guarantee alignment with essential regulatory standards.
In addition, Zephyr excels in test management, facilitating streamlined testing processes that are crucial for maintaining compliance. Furthermore, HP ALM oversees the application lifecycle, fostering collaboration between development and QA teams in regulated settings. Lastly, QTest enhances test case management, promoting teamwork to ensure rigorous adherence to industry regulations. Together, these tools provide a solid foundation for effective regulatory QA practices, ultimately driving compliance and quality assurance success.
- insight7
In understanding insight7, it is crucial to recognize the significance of Regulatory QA Practices within highly regulated environments. These practices are essential for ensuring compliance, quality, and risk management in fields such as pharmaceuticals, healthcare, and finance. Organizations in these sectors must diligently follow regulations to avoid potential legal and financial repercussions.
A key aspect of effective quality assurance is integrating a systematic approach to collect and analyze data. This can include continuous monitoring, meticulous documentation, and regular audits. Establishing a culture of compliance means fostering an environment where team members actively participate in understanding and adhering to regulatory guidelines. By promoting clear communication and collaboration, organizations can more efficiently transform regulatory mandates into actionable insights that drive quality improvements.
Ultimately, adopting robust Regulatory QA Practices not only mitigates risks but also enhances overall operational efficiency, contributing to a more resilient organization.
As the top choice, insight7 offers comprehensive solutions for managing QA in regulatory settings. We will delve into its features that support compliance and efficiency.
In highly regulated environments, maintaining quality assurance is crucial for compliance and operational success. The right solution can significantly enhance QA processes, ensuring they align with established regulations and standards. A comprehensive platform specifically designed for regulatory settings offers features that streamline compliance checks and improve efficiency across various quality assurance activities.
The key functionalities of such a solution include customizable evaluation templates tailored to specific regulatory requirements. These templates allow teams to assess each interaction against defined criteria, ensuring that every detail is scrutinized for compliance. Furthermore, advanced data analysis capabilities help in extracting valuable insights, enabling organizations to monitor their QA performance effectively. By integrating these tools into daily operations, businesses can not only meet regulatory expectations but also enhance the overall quality of their services.
- Jira
Jira plays a crucial role in enhancing Regulatory QA Practices within highly regulated environments. Its effective tracking and management capabilities allow teams to monitor compliance throughout the QA process meticulously. By providing a centralized platform for issue tracking, Jira enables QA professionals to manage both pre-production and post-production tasks, ensuring adherence to regulatory requirements and industry standards.
In addition to facilitating communication among team members, Jira helps create detailed documentation of QA activities. This documentation serves as critical evidence during audits and inspections, showcasing how compliance is maintained throughout the development lifecycle. Furthermore, the platform's customizable workflows can be aligned with specific regulatory frameworks, making it easier for teams to adopt best practices tailored to their unique needs. With Jira in place, organizations can streamline their quality assurance processes while achieving excellent regulatory compliance outcomes.
Known for its robust tracking capabilities, Jira is instrumental in managing QA processes and ensuring project alignment with regulatory standards.
In highly regulated environments, the effectiveness of Regulatory QA Practices is heavily reliant on robust tracking capabilities. Tools such as Jira are indispensable in this context, enabling teams to manage QA processes with precision. With its extensive tracking features, Jira allows for clear visibility into each stage of the QA workflow, ensuring that compliance and quality standards are consistently met across projects.
Using Jira, teams can create and monitor issues, track progress, and report on outcomes, all while aligning with regulatory standards. This systematic approach not only facilitates adherence to compliance but also allows teams to document their processes comprehensively, which is crucial during audits. Moreover, customizable workflows within Jira provide flexibility to tailor processes that adapt to specific industry regulations, ensuring that quality assurance is seamlessly integrated with regulatory requirements throughout the project lifecycle. Thus, effective utilization of Jira can greatly enhance the efficiency and reliability of QA practices in regulated settings.
- Zephyr
Zephyr
In the context of regulatory QA practices, Zephyr stands out as a powerful test management tool. It plays a vital role in facilitating streamlined testing efforts while ensuring compliance in highly regulated environments. By providing comprehensive functionalities, Zephyr helps teams efficiently plan, execute, and track testing activities, which is crucial for aligning with stringent regulatory requirements.
Moreover, Zephyr’s integration capabilities allow seamless collaboration among various stakeholders involved in QA processes. The tool enables real-time visibility into testing progress and generates reports that are critical for audits and compliance checks. By harnessing Zephyr, organizations can enhance their QA practices, reduce risks associated with non-compliance, and ultimately maintain high-quality standards in their products or services. Thus, utilizing Zephyr can significantly improve the overall effectiveness of regulatory QA across different industries.
As an effective test management tool, Zephyr provides solutions to streamline testing efforts and maintain compliance in controlled environments.
In today's regulated industries, maintaining compliance while ensuring comprehensive testing is essential. An effective test management tool can streamline these efforts, providing a systematic approach to quality assurance. Zephyr exemplifies this by offering robust solutions tailored to controlled environments. It allows teams to efficiently organize testing activities, manage test cases, and track compliance requirements seamlessly.
By facilitating real-time collaboration and transparency, Zephyr ensures that all stakeholders are informed and aligned throughout the testing process. This is particularly crucial in regulatory QA practices, where adherence to standards can mean the difference between successful product launches and costly setbacks. Furthermore, its integration capabilities with other tools enhance the overall quality assurance strategy, empowering organizations to maintain compliance and elevate their testing methodologies. By adopting such innovative tools, companies can navigate the complexities of regulatory environments more effectively.
- HP ALM
HP ALM plays a pivotal role in enhancing regulatory QA practices within highly regulated environments. This comprehensive tool not only facilitates the entire application lifecycle management process but also fosters collaboration between development and QA teams. By integrating all stages of development, HP ALM ensures that quality assurance efforts align seamlessly with critical regulatory requirements.
One of the key strengths of HP ALM is its ability to provide thorough documentation and traceability. This feature is essential for maintaining compliance and can significantly reduce the risk of errors during the QA process. Additionally, the tool allows for real-time monitoring of testing activities, which helps to quickly identify and address compliance issues as they arise. Ultimately, HP ALM proves invaluable for organizations striving to achieve excellence in their regulatory QA practices while navigating complex compliance landscapes.
HP ALM supports the entire application lifecycle management process, bringing together development and QA teams to meet strict regulatory requirements.
HP ALM effectively integrates development and QA teams throughout the application lifecycle, ensuring adherence to stringent regulatory requirements. By providing a collaborative platform, this tool fosters communication between these critical groups, enabling swift identification and resolution of issues. In regulated environments, compliance is paramount, and HP ALM offers features designed to help teams maintain high-quality standards while meeting these requirements seamlessly.
This system plays a pivotal role in enforcing Regulatory QA Practices by streamlining documentation, tracking changes, and facilitating audits. It allows teams to incorporate feedback and adjust processes in real time, ensuring that every development phase aligns with compliance mandates. Overall, harnessing HP ALM can significantly enhance the QA process, ensuring that all team efforts converge towards a common goal: delivering reliable and compliant applications in a highly regulated landscape.
- QTest
QTest serves as a robust tool specifically designed to enhance quality assurance in highly regulated environments. Its primary function is to manage test cases effectively, allowing teams to collaborate seamlessly while ensuring adherence to stringent regulatory frameworks. Utilizing QTest can significantly facilitate the documentation and tracking processes critical for Regulatory QA Practices.
One of the standout features of QTest is its ability to integrate with various testing frameworks and tools, streamlining workflows and reducing communication barriers within teams. This integration enables a comprehensive overview of testing activities and compliance metrics, simplifying audits or regulatory inspections. Furthermore, QTest allows for the customization of reporting tools, enabling QA professionals to generate insightful analytics tailored to regulatory requirements.
By leveraging QTest, teams can enhance efficiency, ensure rigorous compliance, and maintain high-quality standards in their QA processes. Hence, QTest serves as a vital asset in successfully navigating the complexities of quality assurance in highly regulated environments.
QTest excels in managing test cases and facilitating collaboration to ensure adherence to regulatory frameworks.
QTest excels in managing test cases and facilitating collaboration to ensure adherence to regulatory frameworks. In highly regulated environments, effective quality assurance is crucial. This platform streamlines the creation, organization, and tracking of test cases, enabling teams to maintain rigorous compliance. By offering an intuitive interface, QTest allows users to easily navigate through complex regulatory requirements, thereby saving time and enhancing productivity.
Collaboration is at the heart of QTest's functionality. The platform encourages team members to communicate seamlessly, sharing insights and updates in real-time. This fosters a cooperative environment where compliance challenges are addressed collectively. With features designed to facilitate transparency, such as audit trails and reporting capabilities, stakeholders can ensure that all regulatory QA practices are upheld. Ultimately, QTest empowers organizations to navigate the intricacies of regulatory frameworks while promoting a culture of quality and accountability.
Conclusion on Embracing Regulatory QA Practices
Embracing Regulatory QA Practices is essential for ensuring compliance and quality in highly regulated environments. Organizations must prioritize understanding regulatory requirements, implementing effective risk management strategies, and utilizing the right tools to streamline their QA processes. This approach not only enhances compliance but also fosters a culture of quality assurance that resonates throughout the organization.
To succeed, companies should continuously revisit their regulatory QA practices, adjusting them to meet evolving standards and expectations. Proactive engagement with these practices will ultimately lead to improved outcomes, demonstrating a commitment to excellence and reliability in all operations. By embracing these principles, organizations can confidently navigate the complexities of regulated environments.
Navigating the complexities of QA in highly regulated environments requires a strategic approach and the right tools at your disposal. By understanding regulatory requirements, managing risks, and leveraging suitable tools, organizations can ensure compliance and maintain high-quality standards.
Effectively navigating the complexities of QA in highly regulated environments involves a strategic approach combined with the right tools. A thorough understanding of regulatory requirements is crucial. Knowing the laws and guidelines that govern an industry helps professionals tailor their QA practices accordingly. This understanding ensures compliance, minimizes the risk of errors, and improves overall quality.
In addition to grasping regulations, managing risks is a critical component of successful QA. Identifying potential vulnerabilities can help teams design appropriate mitigation strategies. Using suitable tools, such as those specifically designed for quality assurance, can streamline the process. These tools not only facilitate compliance but also foster a culture of accountability and accuracy. By integrating knowledge of regulations, risk management, and effective tools, teams can achieve a high standard of quality while confidently navigating the intricacies of regulatory environments.